Aluminum alloy is widely used in our daily life, and vacuum brazing is very important in the processing of some aluminum alloy products. Today, we will talk about the vacuum brazing of aluminum alloys and the materials used for brazing.
Advantages of aluminum alloy
Aluminum alloys are widely used in aerospace, construction, electrical appliances, automobiles, ships, and other sectors for their lightweight, good corrosion resistance, excellent thermal and electrical conductivity, etc. The amount is increasing and has long been considered as a structural material for making heat exchangers, waveguides, and many other complex components.
The processing of aluminum alloys is very important in the manufacture of their products, and one of the most important methods is the vacuum brazing process, which does not require any brazing flux. Because of its many advantages: no complicated cleaning work before and after welding, simplified operation, avoidance of slagging caused by brazing flux, no residual brazing flux in the structure to ensure its corrosion resistance, high productivity, etc., The application of vacuum brazing of aluminum alloys is bound to become more and more widespread in view of the current and future environmental protection concept.
Principle of vacuum brazing of aluminum alloys
The surface of aluminum alloys has a dense and very stable oxide film Al2O3, which is the main obstacle to the wetting of the base material by the melting brazing material. It is difficult to remove the oxide film by vacuum conditions alone and must be accompanied by certain metal activators, such as Mg, Bi, etc. In the early days, it was thought that the purpose of film removal could be achieved by the action of Mg. This is because:
On the one hand, Mg reacts with the residual O2 and H2O in the vacuum to eliminate their harmful effects on aluminum; on the other hand, and most importantly, in reaction equation (3), Mg reacts with Al2O3 on the surface of the base material for the purpose of direct removal of the oxide film. However, a large number of studies have since shown that the oxide film is not completely removed from the base material, so a new viewpoint on the removal of the film was put forward, in which Mg plays a role in eliminating O2 and H2O from the environment, and Mg vapor penetrates into the material layer under the film together with the diffused Si, causing the formation of a low-melting-point Al-Si-Mg alloy on the surface layer and melting it, thus breaking the bond between the oxide film and the base material, allowing the melted brazing material to wet the base material and spread on the base material under the film. The surface film is removed by spreading the base material under the film and floating the surface film.
Although there has been a great deal of research and testing to make vacuum brazing of aluminum alloys common in the industry, no reasonable explanation can be given for the many scrapped brazed joints that occur in actual production. Aluminum alloy vacuum brazing is sensitive to small changes in parameters, and prefabricated brazing alloy cladding from different manufacturers often shows significantly different brazed joints, even though the composition is within the specified range. So far, due to the lack of a truly accurate understanding of the mechanism of vacuum brazing of aluminum alloys, the development of an ideal vacuum brazing process has been greatly limited and is still largely based on the practical experience accumulated in the past.
Brazing alloys for vacuum brazing of aluminum alloys
Most brazing alloys are based on the Al-Si system, w(si) is generally in the range of 7% to 12%. These brazing alloys are rare and excellent in terms of brazeability, strength, and color consistency of the base material, plating, and corrosion resistance, especially because they can be densified to greatly increase the toughness and bending strength of the brazed joint. The Al-Si system with a w(si) of 11.7% is a eutectic system with a eutectic temperature of 577°C. This composition is the standard brazing material commonly used in production and is suitable for brazing a variety of aluminum alloys with relatively high melting points, such as 3A21. The addition of Mg and other elements to the Al-Si braze can be used to formulate new brazing alloys. Commonly used brazing material components and brazing temperature ranges are shown in table 1 below:
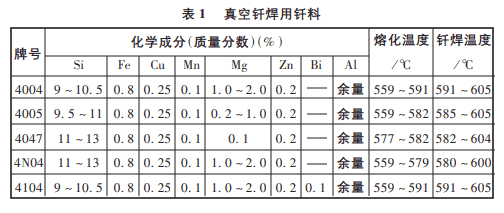
In addition, brazing composite plates is also a commonly used method, i.e., a brazing alloy is wrapped on one or both sides of the structure’s base metal, and the relevant data are shown in table 2 below.
Vacuum brazed laminates are much easier to use than wire, strip or powder, and can be easily used to make complex parts. When the cladding metal on the brazed plate melts, it can directly wet the bonding base material and immediately fill the gap. Joints can be formed with only slight diffusion.
In order to lower the melting point of the aluminum brazing material, the Al-Si-Ge system was developed, which can lower the melting point up to 423°C. The fluidity of the brazing material is good and the spreading is excellent, but the addition of Ge makes the brazing material much more brittle, the corrosion resistance deteriorates, and the color is dark, so it cannot be used as a practical brazing material for vacuum brazing. According to the relevant data, the system alloy has improved greatly after the densification of Na, Sr, La, and other elements, which proves to be a very meaningful brazing alloy with a good brazing process. In-depth studies should lead to a brazing alloy with better overall properties.
The phase diagram of this system shows an inclusion eutectic point with w(Si)=13% and w(Be)=0.5%. The composition taken is the inclusion eutectic point, whose temperature of 571°C is 6°C lower than that of the Al-Si eutectic. The strength of the brazing material is significantly increased by the combined densification of Sr and La. However, the melting point is still high and not suitable for brazing high-strength aluminum alloys such as 6061. If the melting point can be further lowered, the brazing material of this system will be very promising.
At present, although some low melting point brazing alloys for vacuum brazing are available in the laboratory, the real brazing alloys with certain corrosion resistance and good mechanical properties still have not reached the requirements suitable for industrial production.
Check out our Vacuum Brazing Furnace in details
More Vacuum Heat Treatment:
- Vacuum Heat Treatment and Aluminum Processing (1): the defects of traditional vacuum annealing technology
- Vacuum Heat Treatment (1): what is vacuum heat treatment, the feature, how to choose…
- Vacuum Heat Treatment (2): How to choose the vacuum heating temperature and heating time?
Follow us on Facebook